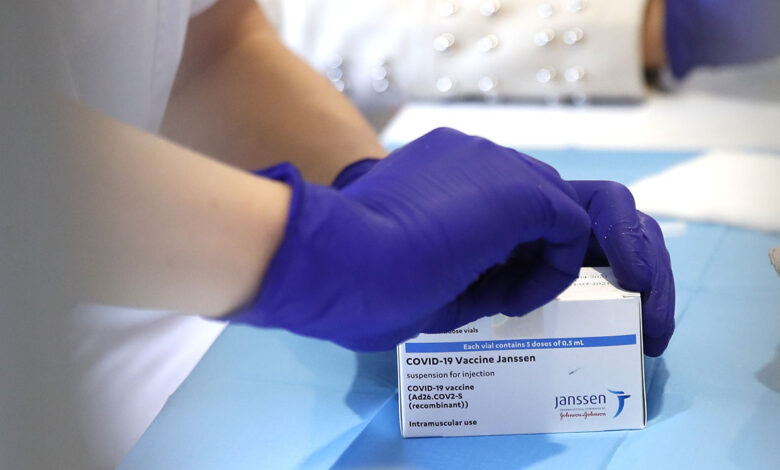
Additional clot cases tied to Johnson & Johnson COVID-19 vaccine: CDC
[ad_1]
An official with the Centers for Disease Control and Prevention (CDC) announced more than two dozen total blood clot cases in individuals who received Johnson & Johnson’s one-shot COVID-19 vaccine amid a backdrop of 8.7 million recipients.
Dr. Tom Shimabukuro with the CDC COVID-19 vaccine task force noted 28 total cases of so-called thrombosis with thrombocytopenia (TTS) following J&J vaccination reported through a national surveillance system, Vaccine Adverse Event Reporting System (VAERS). TTS is a broad term including the rare but potentially deadly cerebral venous sinus thrombosis (CVST), but also deep vein thrombosis and pulmonary thromboembolism.
The update marks an increase from a previously reported 17 instances in late April, when the agency was investigating two new cases of a rare, severe blood clot that occurred alongside low platelets in Johnson & Johnson COVID-19 vaccine recipients, in addition to 15 previously reported TTS cases.
CDC PANEL RECOMMENDS PFIZER COVID-19 VACCINE FOR KIDS 12-15
The federal health agency said the current evidence suggests a possible causal link to the J&J COVID-19 vaccine.
In the update Wednesday, Shimabukuro noted 25 so-called Tier 1 TTS cases (clots in rare, unusual locations, like the brain) and three so-called Tier 2 TTS cases (clots in common locations), as of May 7. All of the patients were vaccinated prior to the national recommended pause on April 13, he said. Most of the patients were women, though six cases were among men.
More specifically, 19 of the 28 cases involved rare but serious blood clotting in the brain called cerebral venous sinus thrombosis, or CVST. None of the patients were pregnant, which can lead to a heightened clotting risk.
Shimabukuro presented data indicating females aged 30 to 39 had the highest reporting rates of TTS at 12.4 cases per million doses administered, though females aged 40 to 49 had the “most pronounced” increase in cases; the reporting rate in this age group is 9.4 clot cases per million doses administered.
No confirmed clotting cases have occurred after more than 135 million administered doses of Pfizer’s COVID-19 vaccine, nor after over 110 million doses administered of Moderna’s jab. Officials say the mRNA vaccines are not associated with an increased clotting risk post-vaccination.
Though more than 1.2 million J&J doses have been administered since the national rollout resumed, CDC officials say the vaccines are being administered to females but in a smaller proportion.
COVID-19 VACCINE DOES NOT DAMAGE PLACENTA IN PREGNANCY, STUDY FINDS
There were no additional deaths since the three reported deaths following the CDC committee’s meeting in April. However, four patients remain hospitalized with one in intensive care, two patients were discharged to a post-acute care facility and 19 patients were discharged home.
The advisory committee on April 23 recommended that the Johnson & Johnson COVID-19 vaccine resume rollout, but include new language on the product’s emergency use authorization (EUA) warning remote risks of serious blood clots.
[ad_2]
Source link