FDA issues alert on hand sanitizer products from Mexico

[ad_1]
The Food and Drug Administration (FDA) has issued an import alert on hand sanitizer products from Mexico amid serious safety concerns. The alert aims to stop the potentially harmful products from crossing U.S. borders before the FDA can review its safety.
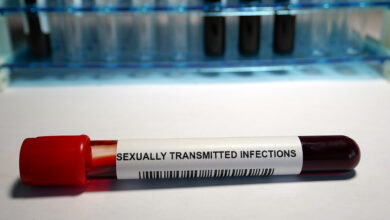
The agency cited concerns over methanol-contamination, which is toxic when the chemical is absorbed through the skin, and life-threatening when ingested.
“Under the import alert, alcohol-based hand sanitizers from Mexico offered for import are subject to heightened FDA scrutiny, and FDA staff may detain the shipment,” reads a statement posted Tuesday. “As part of their entry review, FDA staff will consider any specific evidence offered by importers or manufacturers that the hand sanitizers were manufactured according to U.S. current good manufacturing practice requirements. This marks the first time the FDA has issued a countrywide import alert for any category of drug product.”

Methanol-contaminated hand sanitizer can be toxic when absorbed into the skin, and life-threatening when ingested, the FDA warned. (iStock)
MEXICO’S PRESIDENT TOOK COMMERCIAL FLIGHT HOURS BEFORE COVID-19 DIAGNOSIS: REPORT
The import alert comes amid an uptick in hand sanitizer use during the pandemic, said Judy McMeekin, FDA Associate Commissioner for Regulatory Affairs. She added that the action is necessary to protect a safe supply of alcohol-based hand sanitizers.
The agency’s analyses of the products from Mexico last year found that the vast majority (84%) were non-compliant with regulations. Over 50% of the samples had toxic ingredients, like methanol and 1-propanol, at harmful levels.
THESE HAND SANITIZERS AREN’T MADE WITH ENOUGH ALCOHOL, FDA SAYS
The FDA warned that methanol-contaminated hand sanitizers can lead to blindness, heart and central nervous system effects, hospitalizations and death. People who ingest the products are most at risk, though applying the products on the hands can result methanol poisoning.
The FDA maintains a list of specific hand sanitizers to avoid, which has swelled to over 200 products. Recent Mexico-derived hand sanitizers flagged by the FDA include products from “4E Global SAPI de CV,” among other manufacturers.
“Consumers who have been exposed to hand sanitizer contaminated with methanol and are experiencing symptoms should contact their local poison control center and seek immediate medical treatment for potential reversal of the toxic effects of methanol poisoning,” reads the statement.
[ad_2]
Source link