FDA panel endorses Johnson & Johnson’s COVID-19 vaccine
[ad_1]
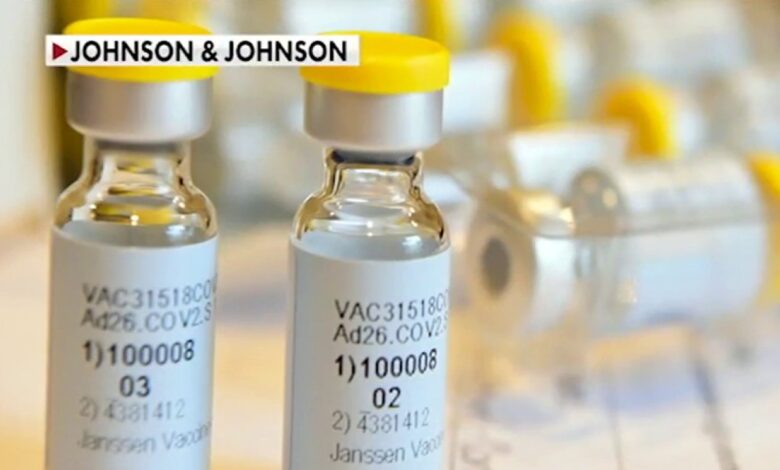
A U.S. Food and Drug Administration advisory panel on Friday voted to endorse Johnson & Johnson’s coronavirus vaccine, clearing the way for FDA leaders to grant emergency use authorization amid the ongoing fight against COVID-19.
The vote was 22-0, with all committee members voting in favor of endorsing the jab for emergency use approval.
The committee was charged with voting on the following question: “Based on the totality of scientific evidence available, do the benefits of the Janssen COVID-19 Vaccine outweigh its risk for use in individuals 18 years of age and older?”
Although significant, the committee’s vote in favor of emergency use authorization (EUA) is not final. The vote will now go before FDA officials who will decide whether to grant EUA based on the committee’s findings. Such approval would come after the regulatory agency granted EUA to both the Pfizer-BioNTech and Moderna jabs late last year.
JOHNSON & JOHNSON SAYS IT CAN PRODUCE 20M DOSES OF COVID-19 VACCINE BY LATE MARCH
The FDA panel’s vote to approve the company’s EUA was expected, as the vaccine was found to have a “favorable safety profile with no specific concerns identified that would preclude issuance of an EUA,” the FDA said in documents posted ahead of Friday’s meeting of the panel of independent experts.
The vaccine candidate — derived from an adenovirus vector formula, unlike the vaccines created by Moderna and Pfizer-BioNTech which were created using mRNA technology — showed to be 66.9% effective against moderate-to-severe disease in a global trial two weeks post-vaccination, per the documents.
HOW DO WE KNOW COVID-19 VACCINES ARE SAFE?
The committee noted no COVID-19-related deaths in vaccinated individuals as of Feb. 5, though seven COVID-19-related deaths occurred in the placebo group. All seven deaths involved individuals at study sites in South Africa with at least one underlying health condition.
“These results suggest that the vaccine is efficacious against mortality associated with COVID-19,” the documents read.
WHAT’S THE DIFFERENCE BETWEEN MRNA VACCINES AND CONVENTIONAL ONES?
However, the committee noted that vaccine efficacy results had “limited interpretability” for those aged over 75 and “certain racial groups.” There was not enough data to assess vaccine efficacy in those previously infected.
The most common reactions associated with the vaccine included pain at the injection site, headache, fatigue and myalgia, which were mostly “mild and moderate,” resolving within two days post-vaccination. Participants ages 18-59 experienced reactions occurring soon after vaccination more often than those aged over 60, per the documents.
CLICK HERE FOR FULL CORONAVIRUS COVERAGE
It’s worth noting that if the FDA clears the J&J shot for U.S. use, it will not boost vaccine supplies significantly right away. Only a few million doses are expected to be ready for shipping in the first week. But J&J told Congress this week that it expects to provide 20 million doses by the end of March and 100 million by summer.
Still, “once approved for emergency use, the J&J vaccine will become the third sharp arrow in our quiver to defeat this awful virus. More production means more people will be vaccinated sooner – to mitigate transmission, quell the pandemic, and reduce future deaths,” Dr. Robert Amler, dean of the School of Health Sciences and Practice at New York Medical College and former Centers for Disease Control and Prevention (CDC) chief medical officer, recently told Fox News.
Fox News’ Kayla Rivas and the Associated Press contributed to this report.
[ad_2]
Source link