FDA updates Moderna COVID-19 vaccine EUA in bid to speed rollout
[ad_1]
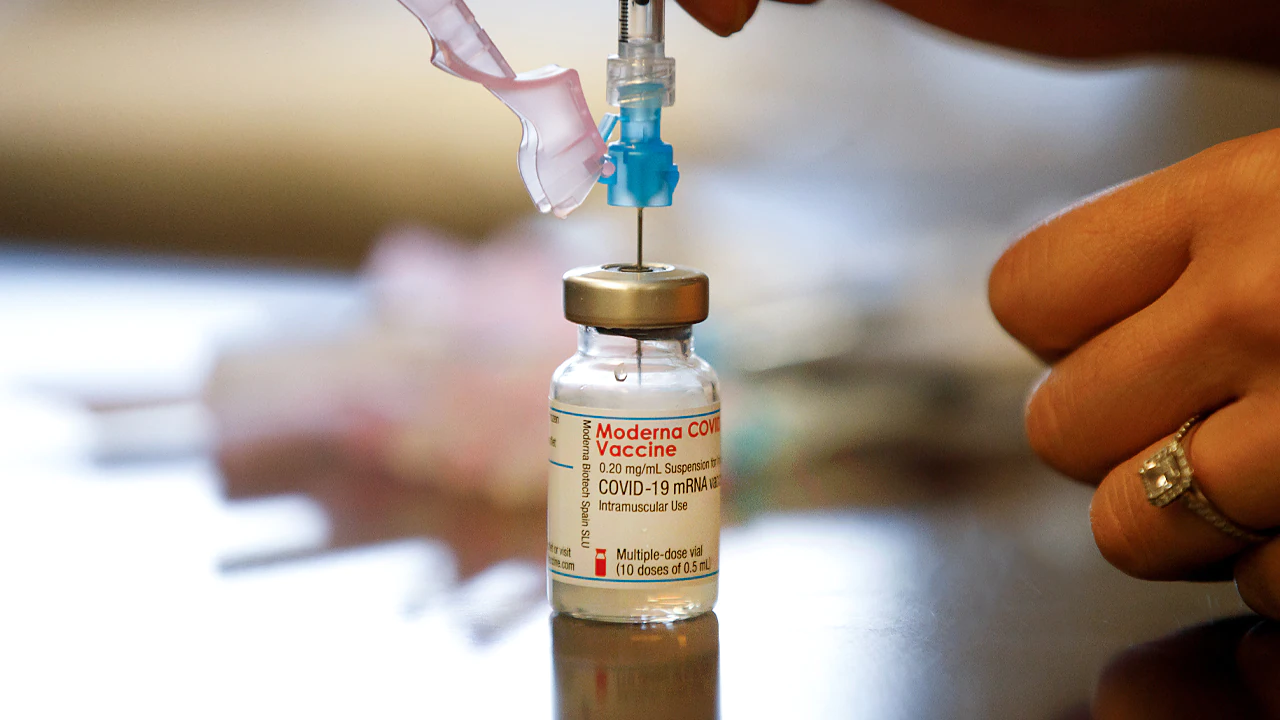
The FDA announced two revisions to the emergency use authorization (EUA) granted to Moderna’s COVID-19 vaccine in the hopes that it will help expedite the rollout. The updates, announced Thursday, clarifies the number of extractable doses per vial for vials currently available is 11, and also authorizes the availability of an additional multi-dose vial that could contain a maximum of 15 doses.
The FDA noted that despite the update, depending on the type of syringes and needles used to extract each dose, there may not be sufficient volume to extract more than 10 doses from the vial containing a maximum of 11 doses, or more than 13 doses from the vial containing a maximum of 15 doses.
As such, the agency warns against pooling remaining vaccine from multiple vials to constitute a full dose.
EARLY TRIALS FOR MODERNA’S COVID-19 VACCINE AGAINST SOUTH AFRICAN VARIANT BEGIN, NIH SAYS
“If one vial becomes contaminated during use, pooling doses from multiple vials can spread contamination to other vials,” the FDA warned. “Use of contaminated vials may cause serious bacterial infections in vaccinated individuals.”
The update also notes that the dosing regimen remains unchanged, and the vaccine will continue being administered as a two-dose series at 0.5 mL in each dose spaced one month apart.
CAN I STILL SPREAD CORONAVIRUS AFTER I’M VACCINATED?
“Both of these revisions positively impact the supply of Moderna COVID-19 Vaccine, which will help provide more vaccine doses to communities and allow shots to get into arms more quickly,” Peter Marcks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research, said in a news release posted Thursday. “Ultimately, more vaccines getting to the public in a timely manner should help bring an end to the pandemic more rapidly.”
According to the CDC, over 71.3 million doses of the Moderna vaccine have been delivered in the U.S. It was the second such COVID-19 to receive EUA in the U.S., following the one granted to Pfizer and BioNTech’s product.
CLICK HERE FOR COMPLETE CORONAVIRUS COVERAGE
Separately, Moderna, in conjunction with the National Institutes of Health, has also begun early-stage trials of an investigative vaccine to protect against the South African coronavirus variant. The trial has begun its Phase 1 clinical trial in adult volunteers and expects to enroll some 210 participants.
[ad_2]
Source link