Mix-and-match COVID-19 vaccine trial results to be available by summer
[ad_1]
Results of a world-first study that will mix and match COVID-19 vaccines are expected by the summer, according to a senior member of the research team.
Volunteers are to be recruited from today for the study, with more than 800 people over the age of 50 being asked to help.
The trial will compare the effect of giving a dose of the AstraZeneca/Oxford vaccine followed a few weeks later by the Pfizer/BioNTech jab – or vice versa.
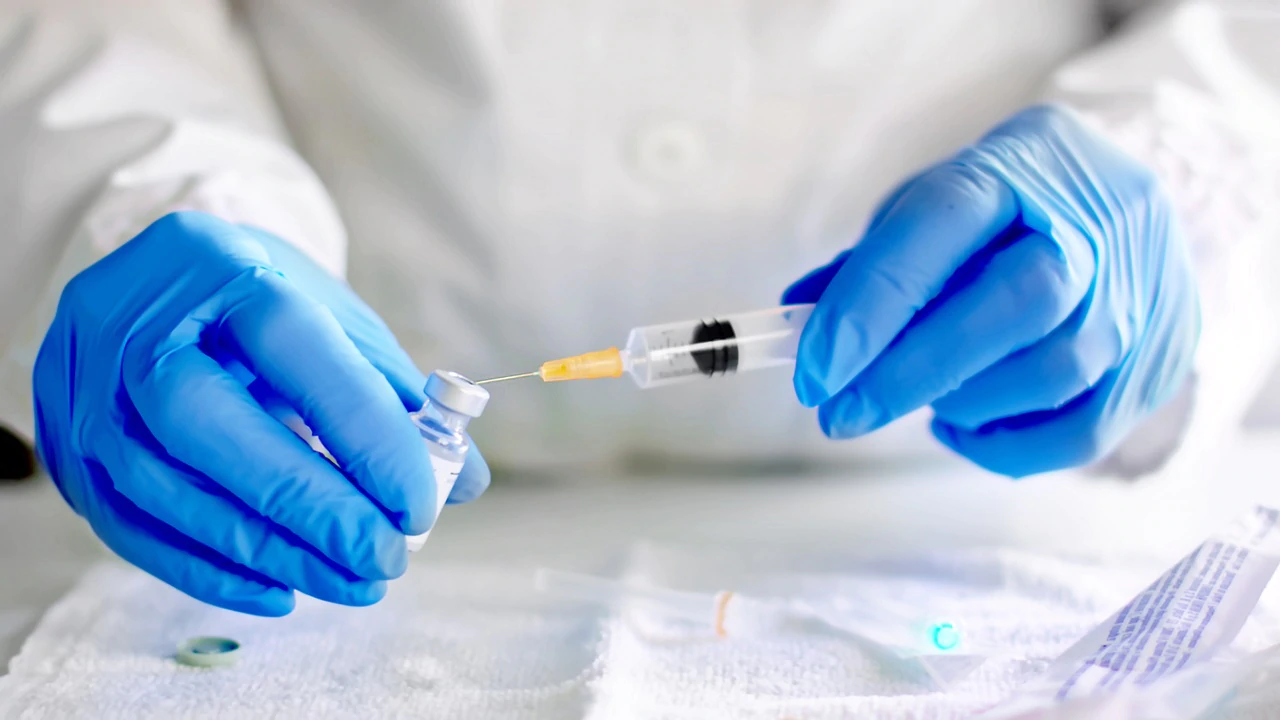
The AstraZeneca-Oxford vaccine has been shown to reduce virus transmission and sustain protection (iStock)
The interval between the doses will be either four or 12 weeks to test whether giving the immune response a longer time to mature can improve the result.
Professor Matthew Snape, a vaccinologist at Oxford University and part of the Oxford vaccine team, told Sky News: “We are looking to unroll the trial this month and then would start to get results through, probably in June or July looking at the antibody levels at least.
“That’s our target and it would be in time to influence the second dose of vaccines for the rollout that’s happening over the next few months.”
ASTRAZENECA-OXFORD COVID-19 VACCINE DROPS VIRUS TRANSMISSION RATE, SUSTAINS PROTECTION: STUDY
The study, run by the National Immunisation Schedule Evaluation Consortium (NISEC), will be run at eight hospital centers around England. Volunteers will be recruited over the next two to three weeks through the COVID-19 Vaccine Research Registry.
They will have regular blood tests to assess their levels of antibodies and T-cells, which help to clear infections and act as an immune memory.
Further vaccines could also be added to the mix once they are approved by the medical regulator.
Prof Snape said the ability to use different jabs would guard against any supply problems.
CASES OF ‘COVID ARM’ FOLLOWING VACCINATION BEING DOCUMENTED BY DERMATOLOGISTS
He said: “If we do show that these vaccines can be used interchangeably in the same schedule this will greatly increase the flexibility of vaccine delivery, and could provide clues as to how to increase the breadth of protection against new virus strains.”
Minister for COVID-19 vaccine deployment Nadhim Zahawi said: “This is a hugely important clinical trial that will provide us with more vital evidence on the safety of these vaccines when used in different ways.”
There are no current plans to alter the current vaccine schedule, which has a gap of up to 12 weeks between two doses of the same jab.
Dr. Peter English, a consultant in communicable disease control at Public Health England, said: “Many vaccines work better if a different vaccine is used for boosting – an approach described as heterologous boosting.
CLICK HERE TO GET THE FOX NEWS APP
“Examples include hepatitis B, where some people respond poorly to vaccination but better if a heterologous booster is given.”
[ad_2]
Source link